Abstract
Acute myeloid leukemia (AML) is a rapidly progressing and highly fatal disease, with only 27% of patients surviving 5 years after diagnosis. Adoptive cell therapy (ACT) provides the opportunity to genetically modify T cells to generate a "living drug" that can recognize and destroy tumor, and subsequently generate a memory population to protect against future recurrence. ACT is particularly attractive as a potential AML therapy, as T cells naturally home to the sites of disease, and ACT has proven effective and is now an FDA-approved therapy for other hematological malignancies. However, several obstacles in the AML bone marrow (AML-BM) can impede T cell therapeutic efficacy, including T cell exhaustion, limited metabolic substrates, and lack of costimulation. Additionally, engineering strategies to improve ACT have mainly been T cell-intrinsic, with little or no support of endogenous anticancer immunity. Costimulatory signals transmitted through cell surface receptors can initiate gene expression programs that address multiple issues in the AML-BM by mechanisms such as lowering the threshold of activation, metabolic reprogramming, and reducing exhaustion. In particular, tumor necrosis family receptor (TNFR) members have been targeted in the clinic with drug-based approaches, with the aim of activating antigen presenting cells (APCs), such as dendritic cells (DCs), macrophages, and B cells, both to boost their own antitumor functions and enhance costimulatory signals leading to enhanced antitumor activity of T and NK cells.
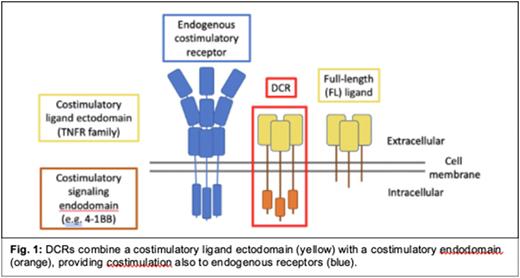
Dual costimulatory receptors as a new cell engineering platform. We innovate synthetic transmembrane fusion proteins (FPs) that combine a receptor ectodomain with a costimulatory signaling endodomain. We have shown that engineered fusion proteins, such as immunomodulatory fusion proteins (IFPs) that utilize an inhibitory decoy ectodomain, can empower T cells to overcome several hurdles faced in the tumor microenvironment and significantly improve therapeutic efficacy in cancer models. Here we report our next generation of FP, called Dual Costimulatory Receptors (DCRs), that pair a costimulatory ligand ectodomain with a costimulatory endodomain. This approach addresses two critical objectives: to enable engineered T cells to attack cancer more powerfully and durably AND to support the activation of endogenous immune cells in the AML-BM. To build on insights from agonist antibody research, we developed DCRs with TNFR ectodomains and created a flexible platform with a variety of pre-selected costimulatory endodomains. We hypothesized that this approach would result in greater efficacy over traditional T cell engineering strategies that aim only to enhance cell-intrinsic function and tested this using murine and human models of AML.
Primary human DCR-engineered T cells exhibited high surface expression of the DCR ectodomain, and increased DCR-T cell activation and ability to lyse tumor in in vitro studies. DCR-T cells co-cultured with immature DCs induced maturation similar to positive control conditions (LPS-maturation), demonstrating cell-extrinsic DCR activity. To determine if promising DCR candidates could enhance in vivo ACT efficacy, AML-bearing B6 mice were treated with1e6 engineered T cells after a single dose of cyclophosphamide (Cy) conditioning therapy, modeling human ACT protocols. Whereas the native full-length costimulatory ligand only modestly improved survival (P=ns), several DCR candidates showed significantly improved therapeutic efficacy (P<0.01-0.05). Here we describe a first-of-kind engineering strategy for the treatment of AML to expand our basic understanding of how different cell-intrinsic and -extrinsic costimulatory signals might achieve enhanced antitumor function and provide a dataset to support clinical translation for AML patients.
Disclosures
Oda:BMS: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.